common name: fir tussock moth
scientific name: Orgyia detrita Guérin-Méneville, 1831 (Lepidoptera: Erebidae: Lymantriinae)
common name: whitemarked tussock moth
scientific name: Orgyia leucostigma (J.E. Smith, 1797) (Lepidoptera: Erebidae: Lymantriinae)
common name: definite tussock moth
scientific name: Orgyia definita Packard, 1864 (Lepidoptera: Erebidae: Lymantriinae)
Introduction - Distribution - Description - Life Cycle and Biology - Host Plants - Medical Importance - Natural Enemies - Control - Cultural Entomology - Selected References
Introduction (Back to Top)
Tussock moths in the genus Orgyia are small moths that are best-known because of their attractive larvae.
Figure 1. Fir tussock moth (Orgyia detrita) caterpillar (dorsal view). Photograph by Donald W. Hall, University of Florida.
In some years the larvae are very numerous and become a problem when they leave their host plants to search for suitable sites to spin their cocoons. Only the three species that are found in Florida will be discussed here. Orgyia detrita (the fir tussock moth) is the most common of the species in Florida followed by Orgyia leucostigma (the whitemarked tussock moth) and finally Orgyia definita (the definite tussock moth), which is rare in Florida (Foltz 2004).
Much of the older literature places the tussock moths in the family Liparidae and more recently in the Lymantriidae. They are now classified in the subfamily Lymantriinae in the family Erebidae (Beadle & Leckie 2012). Orgyia leucostigma was formerly placed in the genus Hemerocampa. For a detailed taxonomic history and synonyms, see Ferguson (1978).
Distribution (Back to Top)
Orgyia detrita: Coastal Plain from Long Island to Florida and Gulf States west to Texas (Ferguson 1978, Wagner 2005, Orgyia detrita entry at North American Moth Photographers Group web site). It is uncommon in the northern parts of its range.
Orgyia leucostigma: Entire eastern U.S. and west to Minnesota and Texas (Ferguson 1978, Orgyia leucostigma entry at North American Moth Photographers Group web site). The form that occurs from South Carolina to Texas is subspecies Orgyia leucostigma leucostigma (Godfrey 1987).
Orgyia definita: Entire eastern U.S. Most common in Northeast and Mid-Atlantic states (Ferguson 1978, Orgyia definita entry at North American Moth Photographers Group web site).
Description (Back to Top)
Larvae: Larvae are 1-1.5 inches in length. They are characterized by hair pencils of black setae that extend forward from the prespiracular verrucae of the prothorax, a dorsal hair pencil of black setae on the eighth abdominal segment, dorsal tussocks on the first four abdominal segments, and mid-dorsal glandular structures on abdominal segments six and seven.
Orgyia detrita has two common color forms in Florida, a dark form and a light form. The sub-dorsal areas (sides) can be a dark gray as in Figures 1 and 2, or they can be light gray to light yellow as in Figure 3. The sides of Orgyia leucostigma are light in color, similar to the light form of detrita. There is a white or yellow line on each side of the dark mid-dorsal line of leucostigma (Ferguson 1978, Godfrey 1987). Orgyia detrita has bright orange spots along the back and sides while the spots on leucostigma are yellow (Foltz 2004).
Detrita and U.S. populations of leucostigma have bright red heads while definita are unique because of their tan or yellow heads. Definita is also lighter in body color than the other two species (Foltz 2004).
The dorsal glandular structures on segments six and seven of leucostigma are bright red, those of detrita are orange, and those of definita are pale yellow.
Figure 2. Fir tussock moth (Orgyia detrita) caterpillar (dorsal view). Photograph by Donald W. Hall, University of Florida.
Figure 3. Fir tussock moth (Orgyia detrita) caterpillar (light form). Photograph by Donald W. Hall, University of Florida.
Figure 4. Fir tussock moth (light and dark forms), Orgyia detrita, and whitemarked tussock moth, Orgyia leucostigma, caterpillars. Photograph by Lyle Buss, University of Florida.
Figure 5. Definite tussock moth (Orgyia definita) caterpillar (front view). Photograph by Lyle Buss, University of Florida.
Figure 6. Definite tussock moth (Orgyia definita) caterpillar (abdomen). Photograph by Lyle J. Buss, University of Florida.
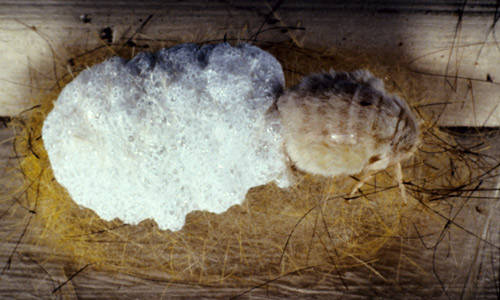
Cocoons & Pupae: Cocoons are constructed of silk and setae from the caterpillars. They are usually found in protected places - in furrows in bark, undersides of limbs, in tree cavities, under loose bark, and often under the soffits of buildings. Also, they are commonly spun in dense masses among the foliage of epiphytic bromeliads (Tillandsia spp.).
Figure 7. Early cocoon of fir tussock moth (Orgyia detrita) before many setae are incorporated. Photograph by Donald W. Hall, University of Florida.
Figure 8. Completed cocoon of fir tussock moth (Orgyia detrita). Photograph by Donald W. Hall, University of Florida.
Figure 9. Orgyia sp. cocoons under eaves of building. Photograph by Donald W. Hall, University of Florida.
Figure 10. Orgyia sp. cocoons among foliage of ballmoss (Tillandsia recurvata). Photograph by Donald W. Hall, University of Florida.
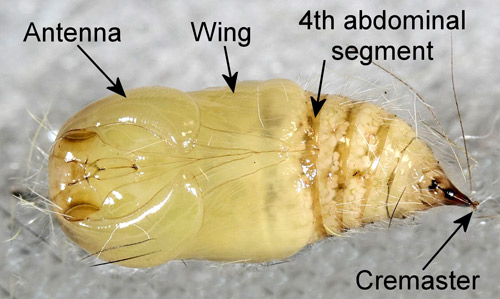
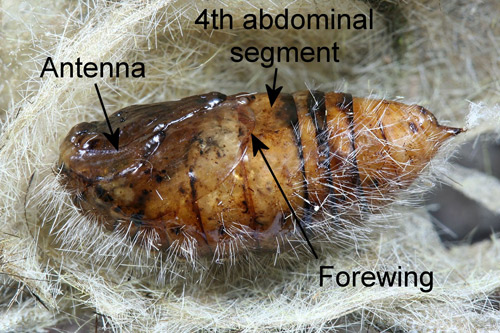
Pupae appear hairy and have patches of dorsal spatulate setae (“vesicles” of Mosher 1916) on abdominal segments 1-3. The antennae of male pupae are longer and broader than those of females and the wings of male pupae are longer than those of female pupae. The wings of female pupae reach only slightly beyond the anterior margin of the fourth abdominal segment while those of male pupae extend nearly to the posterior margin of the segment (Mosher 1916).
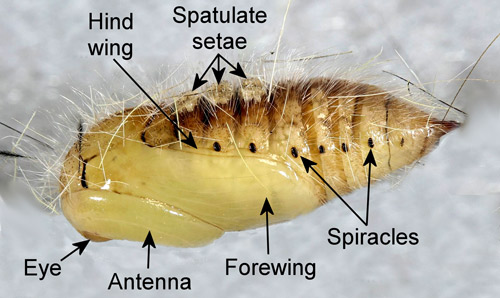
Figure 11. Recently molted male fir tussock moth (Orgyia detrita) pupa (lateral view), Orgyia detrita. Photograph by Donald W. Hall, University of Florida.
Figure 12. Recently molted male fir tussock moth (Orgyia detrita) pupa (ventral view). Photograph by Donald W. Hall, University of Florida.
Figure 13. Mature female fir tussock moth (Orgyia detrita) pupa. Photograph by Donald W. Hall, University of Florida.
Figure 14. Mature tussock moth (Orgyia sp.) pupa with spatulate setae. Photograph by Donald W. Hall, University of Florida.
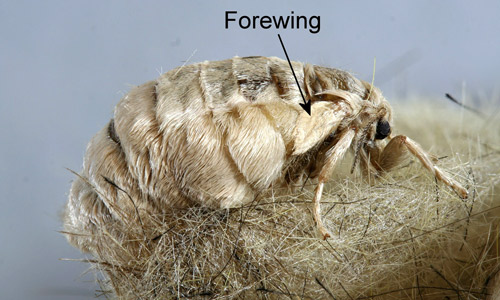
Adults: Adults are dimorphic. Males are small, relatively dull-colored moths with prominent bipectinate antennae. At rest, they hold their first pair of legs in an outstretched position. The genus name Orgyia (Greek for “the length of the outstretched arms” [Borror 1960]), is based on this pose. Wingspreads of Orgyia species are 2.0-3.5 cm (0.78-1.4 in).
For photographs of pinned and spread specimens of males of the Lymantriinae, see Ferguson (1978). Males are difficult to distinguish, but fresh specimens of Orgyia leucostigma and Orgyia definita have a purplish tint that is lacking in Orgyia detrita. Detrita also lacks the whitish tornal spot of leucostigma and definita (Ferguson 1978).
Figure 15. Male fir tussock moth (Orgyia detrita). Photograph by Donald W. Hall, University of Florida.

Figure 16. Male whitemarked tussock moth (Orgyia leucostigma). Note purple tint on wings and white tornal spot. Photograph by Lyle J. Buss, University of Florida.
The literature frequently describes the females as being wingless. Actually, they are brachypterous (short-winged) but cannot fly. At present, females can be identified to species only by association with their respective larvae (or in the case of Florida Orgyia detrita by association with their egg masses).
Figure 17. Female fir tussock moth (Orgyia detrita). Photograph by Donald W. Hall, University of Florida.
Life Cycle and Biology (Back to Top)
Orgyia detrita is univoltine (one generation per year) while the other two species are bivoltine in Florida (Foltz 2006). In Florida, the overwintering eggs begin to hatch in late February. After hatching, the young larvae feed on the remaining egg mass and then spin a silk thread that they use to “balloon” for dispersal (Thurston 2002). Because adult females are flightless, ballooning by young larvae is the major mode of dispersal. Ballooning is also important given their propensity for spinning cocoons off their host plants (i.e., on buildings, fences, and other man-made objects).

Figure 18. Newly-hatched larvae of the fir tussock moth (Orgyia detrita). Photograph by Donald W. Hall, University of Florida.
By the second instar, the larvae are already recognizable because of their short hair pencils. Young larvae eat holes in leaves. Older larvae are leaf-edge feeders.
Figure 19. Second instar fir tussock moth larva (Orgyia detrita). Photograph by Donald W. Hall, University of Florida.
Caterpillars reach maturity and wander in search of sites to spin their cocoons in early April in Florida.
Adults emerge from mid-April to early May. The flightless females remain on their cocoons and release a sex pheromone to attract males. The sex pheromones of Orgyia detrita and Orgyia leucostigma have been characterized (Grant et al. 2003, Gries et al. 2003).
After mating, the females lay a mass of eggs directly on the cocoon and cover them with a protective covering. Detrita and definita females cover their eggs with a secretion and then rub setae from their bodies onto the secretion to form a protective layer over the eggs. Leucostigma females cover their eggs with a frothy secretion but do not cover the secretion with setae (Ferguson 1978). The egg stage is the overwintering stage for all three species.
Figure 20. Female fir tussock moth (Orgyia detrita) applying secretion to her egg mass. Photograph by Donald W. Hall, University of Florida.
Figure 21. Female fir tussock moth (Orgyia detrita) rubbing setae from her abdomen onto her egg mass. Photograph by Donald W. Hall, University of Florida.
Figure 22. Fir tussock moth (Orgyia detrita) cocoon with egg mass covered with setae from female’s abdomen. Photograph by Donald W. Hall, University of Florida.
Figure 23. Female whitemarked tussock moth (Orgyia leucostigma) on egg mass. Photograph by Jerry F. Butler, University of Florida.
Host Plants (Back to Top)
Orgyia detrita: Although the common name is “fir tussock moth”, the only documented hosts are oaks and bald cypress (Taxodium distichum) (Ferguson 1978).
Orgyia leucostigma: Polyphagous. Heppner (2003) listed plants belonging to 116 genera that have been reported as hosts. A few common hosts include oak, cherry, hackberry, and willow.
Orgyia definita: Only willow (Salix sp.) has been confirmed as a host in Florida, but other host plants are also likely (Heppner 2003). Common hosts in other parts of its range include oak, maple, hackberry, birch, and willow (Wagner 2005).
Medical Importance (Back to Top)
The medical importance of Orgyia species caterpillars is well-documented in the scientific (Diaz 2005, Gilmer 1925, Goldman et al. 1960, Knight 1922) and clinical dermatology (Hossler 2009 & 2010 ) literature. Pruritic (itching) dermatitis due to tussock moth caterpillars has been reported to be a problem at child day-care centers and elementary schools in Florida (Atrubin et al. 2012, Atrubin & Granger 2006, Cruse et al. 2007). Contact with the cocoons produces the same symptoms.
The caterpillars may be contacted when they drop from the host trees or when they wander from the trees in search of a place to spin their cocoons. Home owners develop dermatitis from contact with the cocoons while removing them from the soffits of houses. Hairs in the cocoons retain their urticating capability for up to a year or longer.
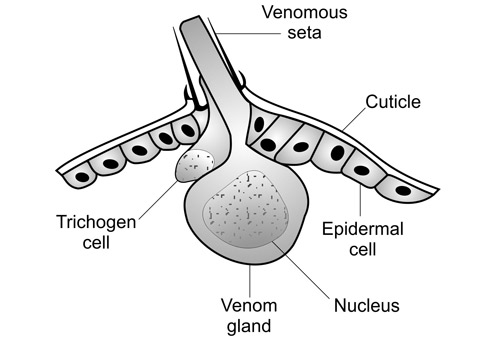
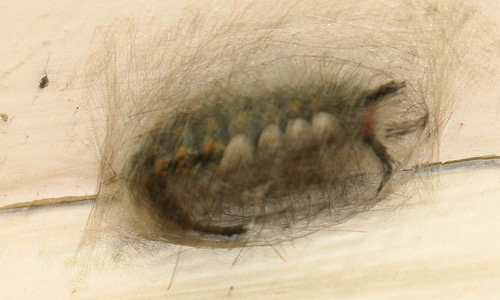
Most of the urticating hairs are in the dorsal tussocks of the caterpillars (Knight 1922), but a few are also found on the lateral verrucae and intermingled with the black plume hairs of the hair pencils (Gilmer 1925). Gilmer (1925) conducted histological studies of the urticating setae of Orgyia leucostigma and found that each seta has a venom gland at its base. The venom has not been adequately characterized.
Figure 24. Tussocks of the fir tussock moth (Orgyia detrita). (Inset: photomicrograph of antrose [distally projecting] barbs on urticating setae of the tussocks). Photograph by Donald W. Hall, University of Florida.
Figure 25. Diagram of urticating seta and associated venom gland of whitemarked tussock moth (Orgyia leucostigma). Redrawn from Gilmer (1925) by Jane C. Medley, University of Florida.
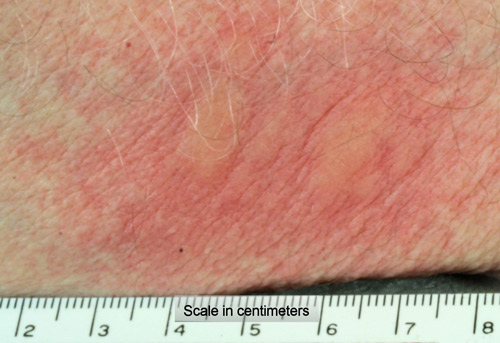
Figure 26. Pruritic welts and erythema resulting from rubbing hairs from the dorsal tussocks of the fir tussock moth (Orgyia detrita) onto the author’s forearm. Photograph by Donald W. Hall, University of Florida.
Welts resulting from contact with Orgyia hairs usually appear within minutes and subside by the next day, but itching and erythema commonly continue for another day or two. People apparently vary somewhat in their sensitivity to Orgyia species hairs. Goldman et al. (1960) studied the histopathology of a sensitive person and reported perivascular concentrations of eosinophils and leucocytes beneath the irritated areas.
Natural Enemies (Back to Top)
Predators: Tussock moth larvae have various natural enemies. Medina and Barbosa (2002) looked at predation of small and large Orgyia leucostigma larvae in a temperate forest and suggested that birds were the major predators of large larvae but most mortality of smaller larvae was probably due to failure to find a suitable host during ballooning dispersal and also possibly to predation by invertebrate predators in the leaf litter. Large ground beetles (Henn et al. 2009) and Polistes paper wasps (Castellanos et al. 2011) have also been reported to attack the larvae up in the trees.
Pathogens: Orgyia caterpillars are infected by nuclear polyhedrosis viruses (Baculovirus) (Cunningham 1972) and cytoplasmic polyhedrosis viruses (Cypovirus) (Hayashi and Bird 1968). Those infected with nuclear polyhedrosis virus typically die in a characteristic pose - hanging limp by their prolegs.
Figure 27. Fir tussock moth caterpillar (Orgyia detrita) exhibiting pose typical of nuclear polyhedrosis virus (Baculovirus) infection. Photograph by Donald W. Hall, University of Florida.
Parasitoids: Larvae and pupae are killed by various parasitoids. Foltz (personal communication) counted egg masses on cocoons and found that there were far less than the 50 percent that would be expected based on a 50:50 ratio of females to males obtained by laboratory rearings. In the year 2000, only 70 of 730 (9.6%) cocoons he examined had egg masses suggesting a high rate of mortality. He has suggested that levels of parasitism of pupae often approach 50 per cent (Foltz 2004, 2006).
Parasitoids of Orgyia detrita and Orgyia definita have not been well-studied, but those of Orgyia leucostigma are well documented. There is little doubt that Orgyia detrita and Orgyia definita also have many parasitoids.
Arnaud (1978, pp. 632-633) listed the following tachinid parasitoids of Orgyia leucostigma: Bessa selecta (Meigen), Carcelia amplexa (Coquillet), Carcelia perplexa Sellers, Carcelia yalensis Sellers, Compsilura concinnata (Meigen), Drino inconspicua (Meigen)*, Euphorocera claripennis (Macquart), Euphorocera edwardsii (Williston), Exorista lobelia Coquillet (currently Nilea lobelia [Coquillet]), Exorista mella Walker, Leshenaultia spp., Lespesia aletiae (Riley), Lespesia frenchii (Williston), Patella leucaniae (Coquillet), Phorocera spp., Sisyropa spp., Winthemia datanae (Townsend), and Winthemia quadripustulata (Fabricius).
*This species is not currently recorded from N.A. north of Mexico (O’Hara 2012).
Names from Arnaud (1978) have been updated by O’Hara and Wood (2004) and O’Hara (2012).
Figure 28. Tachinid puparium from Orgyia sp. larva. Photograph by Donald W. Hall, University of Florida.
The following wasp parasitoids of Orgyia definita and Orgyia leucostigma are recorded by Krombein et al. (1979):
Orgyia definita:
| Apanteles diacrisiae Gahan (Braconidae) | (p. 245) |
| Phobocampe pallipes (Provancher) (Ichneumonidae) | (p. 660) |
| Hyposoter fugitivus (Say) (Ichneumonidae) | (p. 677) |
| Elachertus hyphantriae Crawford (Ichneumonidae) | (p. 980) |
Orgyia leucostigma:
| Bracon xanthonotus Ashmead (Braconidae) | (pp. 168-169) |
| Apanteles acronyctae (Riley) (Braconidae) | (p. 242) |
| Apanteles delicatus Howard (Braconidae) | (p. 245) |
| Apanteles diacrisiae Gahan (Braconidae) | (p. 245) |
| Apanteles hyphantriae Riley* (Braconidae) | (p. 248) |
| Meteorus autographae Muesebeck (Braconidae) | (p. 282) |
| Meteorus hyphantriae Riley* (Braconidae) | (p. 283) |
| Meteorus versicolor (Wesmael) (Braconidae), introduction from Europe | (p. 285) |
| Iseropus coelebs (Walsh)* (Ichneumonidae) | (p. 332) |
| Iseropus stercorator orgyiae (Ashmead) (Ichneumonidae) | (p. 332) |
| Itoplectus conquisitor (Say)* (Ichneumonidae) | (p. 340) |
| Ephialtes annulicornus componotus (Davis) (Ichneumonidae) | (p. 342) |
| Coccygomimus maurus (Cresson) (Ichneumonidae) | (p. 345) |
| Coccygomimus pedalis (Cresson)* (Ichneumonidae) | (p. 345) |
| Theronia atalantae fulvescens (Cresson) (Ichneumonidae) | (p. 347) |
| Gelis insolitus (Howard) (Ichneumonidae), hyperparasite | (p. 406) |
| Gambrus canadensis burkei (Viereck) (Ichneumonidae) | (p. 449) |
| Orgichneumon calcatorius (Thunberg) (Ichneumonidae) | (p. 518) |
| Casinaria limenitidis (Howard) (Ichneumonidae) | (p. 637) |
| Phobocampe pallipes (Provancher) (Ichneumonidae) | (p. 660) |
| Psychophagus omnivorus (Walker) (Pteromalidae), introduction from Europe | (p. 807) |
| Habrocytus thyridopterigis Howard (Pteromalidae) | (p. 813) |
| Tritneptis hemerocampae Viereck (Pteromalidae) | (p. 825) |
| Syntomosphyrum esurus (Riley) (Eulophidae) | (p. 1004) |
| Syntomosphyrum orgyiazele Burks (Eulophidae) | (p. 1005) |
| Telenomus dalmani (Ratzeburg) (Scelionidae) | (p. 1168) |
| *Listed range includes Florida. |
Figure 29. Fir tussock moth caterpillar (Orgyia detrita) parasitized by wasps. The parasitoid cocoons are cloaked by the silk covering (spun by the wasp larvae) beneath the parasitized caterpillar (Inset: parasitoid cocoons from under silk covering - wasps have already emerged). Photographs by Donald W. Hall, University of Florida.
Control (Back to Top)
Control of the caterpillars is difficult because by the time they are migrating from the trees, it is too late. In Florida, feeding damage to large trees by Orgyia species does not usually harm the trees. However, they may occasionally be sufficiently numerous to completely defoliate large trees. Also, large numbers of larvae blown onto small landscape trees may result in severe defoliation.
Figure 30. Large live oak tree defoliated by fir tussock moth (Orgyia detrita) caterpillars. Photograph by Lyle J. Buss, University of Florida.
If control measures are required, chemical insecticide or Bacillus thuringiensis applications recommended for control of other caterpillars should be effective. For current control recommendations, contact your county extension agent.
Cultural Entomology (Back to Top)
Insects are very popular in human culture. Images of butterflies and moths are common in movies, art, jewelry, and fabrics. Although the fir tussock moth is not a highly familiar moth even to most entomologists, an image of an adult male does appear in a popular design used on ornamental paper, wall art, journal covers, purses, and fabric (Tim Holtz, personal communication).
Figure 31. Fabric with image of Orgyia detrita male. © ECLECTIC ELEMENTS (PWTH004.TAUPE Butterflight). Tim Holtz.com. Used with permission.
Selected References (Back to Top)
- Arnaud PH. 1978. A Host-Parasite Catalog of North American Tachinidae (Diptera). United States Department of Agriculture Miscellaneous Publication 1319. Washington, D.C. (21 March 2020)
- Atrubin D, Granger K. April 28, 2006. Contact dermatitis in daycare facilities. EPI-NOTES Disease Surveillance Newsletter. Hillsborough County (Florida) Health Department.
- Atrubin D, Wansbrough L, Cruse K, Stanek D, Blackmore C. 2012. Caterpillar-associated rashes in children - Hillsborough County, Florida, 2011. Morbidity and Mortality Weekly Report 61(12): 209-212. (21 March 2020)
- Beadle D, Leckie S. 2012. Petersen Field Guide to Moths of Northeastern North America. Houghton Mifflin Publishing Company. New York, New York. 611 pp.
- Borror DJ. 1960. Dictionary of Word Roots and Combining Forms. Mayfield Publishing Company. Palo Alto, California. 134 pp.
- Castellanos I, Barbosa P, Caldas A. 2011. Dropping from host plants in response to predators by a polyphagous caterpillar. Journal of the Lepidopterists Society 65(4): 270-272.
- Cruse K, Atrubin D, Loyless T. 2007. Rash illness outbreaks at daycare facilities associated with the tussock moth caterpillar, April 2004 and April 2005. Florida Journal of Environmental Health 195: 14-17.
- Cunningham JC. 1972. Preliminary studies of the nuclear-polyhedrosis viruses infecting the white-marked tussock moth, Orgyia leucostigma. Information Report. Insect Pathology Research Institute. Department of the Environment. Canadian Forestry Service. Sault Ste. Marie, Ontario, Canada. 23 pp. + figures. (17 February 2014)
- Diaz JH. 2005. The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming. American Journal of Tropical Medicine and Hygiene 72(3): 347-357.
- Ferguson DC. 1978. The Moths of America North of Mexico Including Greenland. Fascicle 22.2 Noctuoidea: Lymantriidae. E.W. Classey, Ltd. London. 110 pp.
- Foltz JL. 2004. Tussock moth caterpillars in Florida. (21 March 2020)
- Foltz JL. 2006. Tussock moth caterpillars in north central Florida. Integrated Pest Management Florida. IFAS Extension. University of Florida. (21 March 2020)
- Gilmer PM. 1925. A comparative study of the poison apparatus of certain lepidopterous larvae. Annals of the Entomological Society of America 18: 203-239.
- Godfrey GL. 1987. Lymantriidae. In Stehr FW. Immature Insects. Kendall/Hunt Publishing Company. Dubuque, Iowa. pp. 544-548.
- Goldman L, Sawyer F, Levine A, Goldman J, Goldman S, Spinanger B. 1960. Investigative studies of skin irritations from caterpillars. Journal of Investigative Dermatology 34(1): 67-79.
- Grant GG, Slessor KN, Wei L, Abou-zaid MM. 2003. (Z,Z)-6,9-heneicosadien-11-one, labile sex pheromone of the whitemarked tussock moth. Journal of Chemical Ecology 29(3): 589-601.
- Gries R, Khaskin G, Khaskin E, Foltz JL, Schaefer PW, Gries G. 2003. Enantiomers of (Z,Z)-6,9-heneicosadien-11-ol: Sex pheromone components of Orgyia detrita. Journal of Chemical Ecology 29(10): 2201-2212.
- Hayashi Y, Bird HT. 1968. Properties of a cytoplasmic-polyhedrosis virus from the white-marked tussock moth. Journal of Invertebrate Pathology 12(1): 140.
- Henn T, Weinzierl R, Koehler PG. 2009. Beneficial Insects and Mites. ENY-276. IFAS Extension. University of Florida. Gainesville, Florida. 15 pp. + Figures.
- Heppner JB. 2003. Lepidoptera of Florida. Part 1. Introduction and Catalog. Volume 17 of Arthropods of Florida and Neighboring Land Areas. Division of Plant Industry. Florida Department of Agriculture and Consumer Services. Gainesville, Florida. 670 pp.
- Hossler EW. 2009. Caterpillars and Moths. Dermatologic Therapy 22: 353-366.
- Hossler EW. 2010. Caterpillars and Moths. Part II. Dermatologic manifestations of encounters with Lepidoptera. Journal of the American Academy of Dermatology 62(1): 13-28.
- Knight HH. 1922. Observations on the poisonous nature of the white-marked tussock-moth (Hemerocampa leucostigma Smith and Abbott). The Journal of Parasitology 8(3): 133-135.
- Krombein KV, Hurd PD, Jr., Smith DR, Burks BD. 1979. Catalog of Hymenoptera in America North of Mexico. Volume 1. Symphyta and Apocrita (Parasitica). Smithsonian Institution Press. Washington, D.C. 1198 pp.
(February 6, 2014) - Medina RF, Barbosa P. 2002. Predation of small and large Orgyia leucostigma (J.E. Smith) (Lepidoptera: Lymantriidae) larvae by vertebrate and invertebrate predators. Environmental Entomology 31: 1097-1102.
- Mosher E. 1916. A classification of the Lepidoptera based on characters of the pupae. Bulletin of the Illinois State Laboratory of Natural History 12:17-159. (21 March 2020)
- O’Hara JE, Wood DM. 2004. Catalogue of the Tachinidae (Diptera) of North America north of Mexico. Associated Publishers. Gainesville, Florida. 410 pp.
- O’Hara JE. 2012. Update of Tachinid Names in Arnaud (1978). (Last update: 3 December 2013) (21 March 2020)
- Orgia detrita entry at North American Moth Photographer’s Group. Mississippi Entomological Museum, Mississippi State University, Starkville, MS.
(21 March 2020) - Orgia definita entry at North American Moth Photographer’s Group. Mississippi Entomological Museum, Mississippi State University, Starkville, MS.
(21 March 2020) - Orgia leucostigma entry at North American Moth Photographer’s Group. Mississippi Entomological Museum, Mississippi State University, Starkville, MS. (21 March 2020)
- Thurston GS. 2002. Orgyia leucostigma (J.E. Smith) whitemarked tussock moth (Lepidoptera: Lymantriidae). pp. 201-203. In: Mason PG, Huber JT, eds., Biological Control Programmes in Canada, 1981-2000. CABI Publishing. New York. 583 pp.
- Wagner DL. 2005. Caterpillars of Eastern North America. Princeton University Press. Princeton, New Jersey. 512 pp.















![Tussocks of the fir tussock moth (Orgyia detrita). (Inset: photomicrograph of antrose [distally projecting] barbs on urticating setae of the tussocks).](tussock_moths24.jpg)