common name: hydrellia fly parasitic wasp (unofficial common name)
scientific name: Trichopria columbiana Ashmead (Insecta: Hymenoptera: Diapriidae)
Introduction - Synonymy - Distribution - Description - Life Cycle - Hosts - Damage - Importance for Biological Control - Monitoring and Management - Selected References
Introduction
Trichopria columbiana (Ashmead) (Insecta: Hymenoptera: Diapriidae) is a native endoparasitic wasp. The wasp is a parasitoid of Hydrellia species (Insecta: Diptera: Ephidridae), with multiple implications to biological control. The hydrellia flies are a diverse group with varied ecological roles. Deonier (1971) described 57 Hydrellia species in the Nearctic region. The adults of this genus are semi-aquatic and the immatures are aquatic, feeding on aquatic and semi-aquatic plants (Deonier 1971). Of these 57 species, at least 7 have been described as hosts for Trichopria columbiana. In addition, the wasp has successfully moved from its native hosts to exotic Hydrellia species (Hydrellia pakistanae Deonier and Hydrellia balciunasi Bock) that were imported into Florida for biological control of hydrilla, Hydrilla verticillata (L.f.) Royle, which is widely regarded as one of the worst invasive weeds worldwide (Holm et al. 1997). Deonier (1971) reported that Trichopria columbiana and other parasitic Hymenoptera can negatively impact population densities of Hydrellia spp., especially in certain marginal habitats and when parasitoid population densities are high.
Synonymy
According to Hymenoptera Online, there is only one junior synonym for Trichopria columbiana (Johnson 2014):
Diapria columbiana Ashmead 1893
Distribution
Trichopria columbiana is widely distributed in North America (Bennett 2008) and has been reported from the District of Columbia, Virginia (Ashmead 1893), Michigan (Berg 1950), California (Grigarick 1959), Minnesota (Deonier 1971), Alabama (Grodowitz et al. 1997), Florida (Wheeler and Center 2001), and Texas (Doyle et al. 2002).
Description (Back to Top)
The description of the life stages of this species has been modified from Coon et al. (2014).
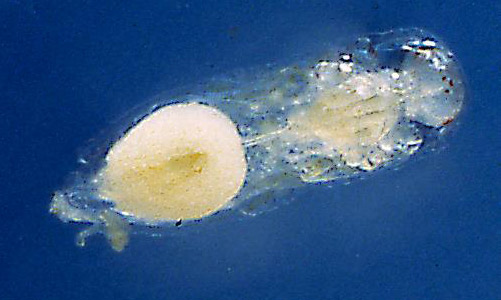
Eggs: Eggs were dissected from Trichopria columbiana ovaries to determine their pre-oviposition morphology. Trichopria columbiana eggs, which are hymenopteriform (wasp-like) in shape, were 0.19 mm long and 0.06 mm wide. The chorion (outer membrane) is smooth and thin. As the chorion is transparent, the developing embryo is clearly visible (Figure 1). The second inner membrane, which is likely to be the vitelline membrane, is flexible. A double-membrane egg is characteristic of hydropic eggs (DeBach 1964). Hydropic eggs take up nutrients and water from the host’s hemolymph for continued development and typically expand in size (Flanders 1950). Hydrellia pakistanae pupae were dissected, and Trichopria columbiana eggs were removed from the host 72 hours post-oviposition. These eggs were much larger than those dissected from the female parasitoid; they measured 0.57 mm in length by 0.28 mm in width.
Figure 1. Egg of Trichopria columbiana (Ashmead). Photograph by Byron Coon, Argosy University.
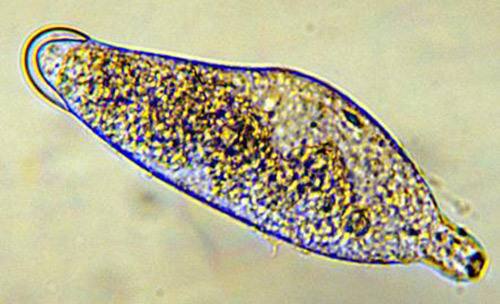
Larvae: There are three instars; the first instar is 0.49 mm long and 0.14 mm wide. At this stage, the body is segmented and the mandibles are large and sclerotized (hardened). The end of the abdomen has a two-lobed appendage with several teeth on each lobe (Figure 2). This instar moves freely in the hemolymph of the host and is believed to obtain oxygen by diffusion.
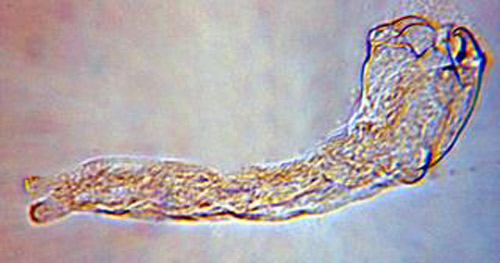
The second instar is 0.92 mm long and 0.31 mm wide, and the third instar is 1.50 mm long and 0.52 mm wide. Both the second and third instars are similar in appearance and are grub-like (Figure 3). The abdominal appendage and large mandibles present on the first instar are absent. The head of the later instars has indistinct mouthparts that are not differentiated from the body. The second and third instars obtain oxygen from the host by attaching to the host tracheal system.
Figure 2. First instar larva of Trichopria columbiana (Ashmead). Photograph by Byron Coon, Argosy University.
Figure 3. Second or third instar larva of Trichopria columbiana (Ashmead). Photograph by Byron Coon, Argosy University.
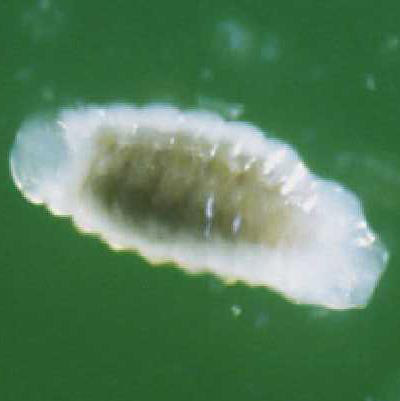
Pupae: The pupae are enclosed in a thin case (Figure 4), which is believed to be the last larval exuvium (cast skin). The case is transparent, and the developing adult is visible inside with the red eyes particularly noticeable. Also visible are many small globules, which are believed to be the meconium (fecal material) released by the last instar before pupation.
Figure 4. Pupa of Trichopria columbiana (Ashmead). Photograph by Byron Coon, Argosy University.
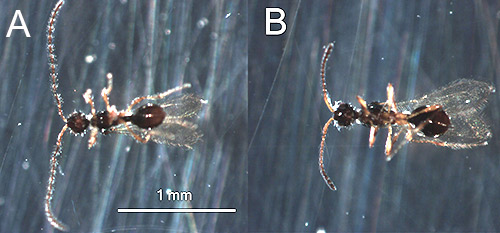
Adult: The following description of the adult stage is based on Ashmead (1893). The overall length of the adult wasp is 1-2 mm. The body is shiny and black in color with the base of the antennae and the legs reddish yellow (Figure 5). The head is round and narrows behind the eyes. The thorax narrows anteriorly forming a round neck. The abdomen is oval-shaped. The wings are strongly fringed and clear with pale yellow veins.
Male and female Trichopria columbiana can be distinguished easily by the shape of the antennae. The antennae of females (Figure 5B) have 12 segments and are slightly clavate or club-like. In contrast, the antennae of males (Figure 5A) have 13 segments and are filiform or thread-like.
Figure 5. Adults of Trichopria columbiana Ashmead; male (A) with filiform or thread-like antennae and female (B) with slightly clavate or club-like antennae. Photographs by Nathan Harms, U.S. Army Engineer Research and Development Center.
Life Cycle (Back to Top)
The life cycle and biology of Trichopria columbiana were studied in detail by Coon et al. (2014) and are summarized below. Four behaviors associated with host location were observed in the laboratory: 1) searching, 2) stem examination, 3) oviposition, and 4) grooming and/or resting. When searching for a host, Trichopria columbiana need to access the pupae of Hydrellia species, which are usually underwater. The female inserts her antennae into the water first, presumably to detect chemical cues of plant damage or the host insect. When given a choice of hydrilla with Hydrellia pupae and hydrilla with Hydrellia larvae, 96% of parasitoids selected the hydrilla with Hydrellia pupae. Therefore, a suitable host in the appropriate life stage is located using chemoreception. The adult female wasp swims underwater by trapping a bubble of air under her wings, which she uses to breathe. After locating a suitable host, the female inserts her ovipositor into the thorax of the fly, which is close to the cuticle of the puparium. The female parasitoid prefers to lay her eggs in early- to intermediate-stage pupae. However, eggs may also be laid in late instars (Grodowitz et al. 2009).
Dissections of both Hydrellia pakistanae and Hydrellia balciunasi revealed that Trichopria columbiana deposits a maximum of three eggs per host. Each female may lay 14 to 32 eggs in a lifetime. The female deposits her eggs directly into the host hemolymph, and the eggs develop in 1 to 3 days. Development of the first instar requires 1 to 3 days, and only a single larva survives. Apparently, the surviving larva uses its mandibles to kill its siblings, thereby avoiding competition for resources. The estimated stadial lengths (time between molts) for the second and third instars is 2 to 5 and 5 to 8 days, respectively. Development of the larval stage is completed in 13 to 23 days. The pupal stage of Trichopria columbiana lasts between 5 and 7 days. After pupation, the adult parasitoid exits the host, which is usually below the water surface, by cutting a hole in the end of the puparium that is not attached to the tracheal system with its mandibles. The adult parasitoid floats to the surface with an air bubble attached to hairs on its abdomen. The bubble of air is believed to have been acquired from the internal environment of the host puparium. This assumption is based on the fact that Hydrellia fly adults exit their puparia in a similar way but ascend to the surface enclosed within an air bubble obtained from inside their puparium (Balciunas et al. 2002). Total development time from egg to adult was on average 22 days (14 to 26 days) in the laboratory at 25 °C. Trichopria columbiana overwinters as an adult in hydrilla at the edge of the water body. The sex ratios that have been recorded in Florida and Texas are female-biased with males being relatively rare. Collection of adults from hydrilla resulted in 14,776 individuals, of which only four were male, a sex ratio of 1: 3694 (male: female).
Hosts (Back to Top)
According to Deonier (1971), Trichopria columbiana attacks at least seven native Hydrellia spp. including Hydrellia ascita Cresson, Hydrellia bergi Cresson, Hydrellia cruralis Coquillett, Hydrellia griseola (Fallén), Hydrellia ischiaca Loew, Hydrellia luctuosa Cresson, and Hydrellia pulla Cresson. This parasitoid also attacks the two Hydrellia spp., Hydrellia pakistanae (Cuda et al. 1997) and Hydrellia balciunasi (Grodwitz et al. 1997), that were introduced in the U.S. for biological control of the aquatic weed hydrilla.
Damage (Back to Top)
The parasitoid lays eggs in the pupae of Hydrellia spp. Once the larvae hatch and begin feeding, the developing Hydrellia pupa provides the food source for the larvae. The developing pupa is killed and will not develop into an adult fly. Therefore, Trichopria columbiana can reduce populations of Hydrellia species including hydrilla leaf mining flies.
Importance for Biological Control (Back to Top)
Trichopria columbiana is a parasitoid of Hydrellia fly species. Depending on the ecological role of the host species, Trichopria columbiana can have a positive or negative effect on biological control.
Some Hydrellia species, including the introduced biological control agents Hydrellia pakistanae and Hydrellia balciunasi, feed on the invasive aquatic weed hydrilla, Hydrilla verticillata. After its introduction into the U.S. by the aquarium industry in the 1950s (Langeland 1996), various control methods, including biological control, were developed and used to manage infestations. Classical biological control studies were initiated in the 1970s (Buckingham 1994). These efforts led to the release of four insects in the U.S., two of which were the leaf-mining ephydrid flies, Hydrellia pakistanae and Hydrellia balciunasi (Center et al. 1990). Despite successful establishment and range expansion of the Asian hydrilla leaf mining fly, Hydrellia pakistanae, population levels of the insect and associated plant damage have remained low (Cuda et al. 2008). However, there is some evidence that past declines of hydrilla in Florida and Texas were associated with local increases in Hydrellia fly populations (Grodowitz et al. 2004). Several abiotic and biotic factors have been identified that could adversely affect Hydrellia pakistanae populations on a landscape scale (Cuda et al. 2008). One of the potentially limiting biotic factors is parasitism by the native endoparasitic wasp Trichopria columbiana.
In Florida, the highest parasitoid activity was recorded in the cooler winter months, October to January, with a peak in January. The average parasitism rate in Florida and Texas was around 20-30% (Coon et al. 2014; Grodowitz et al. 2009). Buckingham and Okrah (1993) concluded that parasitism of the introduced Hydrellia pakistanae and Hydrellia balciunasi by parasitoids of native Hydrellia spp. could be more problematic than interspecific competition between the two introduced biological control agents. Hence, they suggested that parasitism should be carefully monitored. In hindsight, attack of the two introduced Hydrellia spp. by Trichopria columbiana or other parasitoids of native Hydrellia spp. was predictable because the biocontrol agents were not released in an ‘enemy-free space’ (Lawton 1985). When weed biological control practitioners select agents, they should carefully consider the potential for insects to acquire novel parasitoids. This precaution will help avoid reducing biological control agent effectiveness and apparent competition, particularly where species interact through shared natural enemies.
The parasitoid also has been found in Hydrellia pulla pupae, with 63% of puparia (out of a sample of 61 puparia) being parasitized during a summer in Minnesota (Deonier 1971). Hydrellia pulla feeds on pondweeds (Deonier 1971), such as the large-leaved pondweed (Potamogeton amplifolius Tuck.), variable-leaf pondweed (Potamogeton gramineus L.), and Richardson’s pondweed (Potamogeton richardsonii [Benn.] Rydb.), which are all classified as endangered or threatened in their native ranges in the U.S. For this reason, the parasitoid may protect native pondweeds from herbivory by Hydrellia.
On the other hand, Trichopria columbiana is a biological control agent itself, providing control of agricultural pests of rice crops. Grigarick (1959) observed 60% parasitism in one sample of Hydrellia griseola mining rice plants in California. In that same study, low parasitism of the first generation of Hydrellia griseola was observed, but parasitism approached almost 90% in succeeding generations. Deonier (1971) found 38% parasitism by Trichopria columbiana and other parasitoids in 132 puparia of Hydrellia ischiaca, a pest of wild rice crops.
Monitoring and Management (Back to Top)
Adult parasitoids can be extracted from plant material using Berlese funnels. Hydrellia pupae can be dissected or isolated and placed in rearing containers to determine parasitism rates.
The authors would like to acknowledge funding provided by the USDA NIFA RAMP Grant 2010-02825 that helped pay for the production of this article. The authors would like to acknowledge the reviewers that provided feedback on an early draft of the article, Dr. William Overholt and Dr. Verena Lietze.
Selected References (Back to Top)
- Ashmead WH. 1893. A monograph of the North American Proctotrypidae. Bulletin of the United States National Museum 45: 1-472.
- Balciunas JK, Grodowitz MJ, Cofrancesco AF, Shearer JF. 2002. Hydrilla. In Van Driesche R, Blossey B, Hoddle M, Lyon S, Reardon R (editors). Biological Control of Invasive Plants in the Eastern United States, USDA Forest Service Publication FHTET-2002-04. Morgantown, WV.
- Bennett AMR. 2008. Aquatic hymenoptera. In Merritt RW, Cummins KW, Berg MB (editors). An Introduction to the Aquatic Insects of North America, 4th edition. Kendall Hunt Publishing Company, Dubuque, IA.
- Berg CO. 1950. Hydrellia (Ephydridae) and some other acalpytrate Diptera reared from Potamogeton. Annals of the Entomological Society of America 43: 374-398.
- Buckingham GR. 1994. Biological control of aquatic weeds. In Rosen D, Bennett FD, Capinera JL (editors). Pest Management in the Subtropics: Biological Control - a Florida Perspective. Intercept Limited, Andover, United Kingdom.
- Buckingham GR, Okrah EA. 1993. Biological and host range studies with two species of Hydrellia (Diptera: Ephydridae) that feed on hydrilla. Technical Report A-93-7, United States Army Corps of Engineers Waterways Experiment Station, Vicksburg, MS.
- Center TD, Cofrancesco, AF, Balciunas JK. 1990. Biological control of wetland and aquatic weeds in the southeastern United States. In Proceedings of the VII International Symposium on Biological Control of Weeds, 6-11 March 1988, ed. E.S. Delfosse. Istituto Sperimentale per la Patologia Vegetale, Ministero dell’Agricoltura e delle Foreste, Rome, Italy.
- Coon BR, Harms NE, Cuda JP, Grodowitz MJ. 2014. Laboratory biology and field population dynamics of Trichopria columbiana (Hymenoptera: Diapriidae), an acquired parasitoid of two hydrilla biological control agents. Biocontrol Science and Technology 24: 1243-1264.
- Cuda JP, Fox AM, Habeck DH. 1997. Evaluation of biocontrol insects on hydrilla. In Stocker RK (editor). Final Report: Control Technologies for Use Against the Submersed Aquatic Weeds Hydrilla and Hygrophila. Center of Aquatic Plants, UF/IFAS, Gainesville, FL.
- Cuda JP, Charudattan R, Grodowitz MJ, Newman RM, Shearer JF, Tamayo ML, Villegas B. 2008. Recent advances in biological control of submersed aquatic weeds. Journal of Aquatic Plant Management 46: 15-32.
- DeBach P. 1964. Biological Control of Insect Pests and Weeds. Chapman and Hall, London. United Kingdom.
- Deonier DL. 1971. A systematic and ecological study of Nearctic Hydrellia (Diptera: Ephydridae). Smithsonian Contributions to Zoology 68: 1-147.
- Doyle RD, Grodowitz M, Smart RM, Owens C. 2002. Impact of herbivory by Hydrellia pakistanae (Diptera: Ephydridae) on growth and photosynthetic potential of Hydrilla verticillata. Biological Control 24: 221-229.
- Flanders SE. 1950. Regulation of ovulation and egg disposal in the parasitic Hymenoptera. Canadian Entomologist 82: 134-40.
- Grigarick AA. 1959. Bionomics of the rice leaf miner, Hydrellia griseola (Fallen), in California (Diptera: Ephydridae). Hilgardia 29: 1-80.
- Grodowitz MJ, Nachtrieb JG, Harms N, Swindle R, Snell C. 2009. Parasitism and host selection behavior of Trichopria columbiana Ashmead and its effects on establishment and dynamics of Hydrellia spp. populations. ERDC/TN APCRB-BC-15, United States Army Engineer Research and Development Station, Vicksburg, MS.
- Grodowitz MJ, Smart M, Doyle RD, Owens CS, Bare R, Snell C, Freedman J, Jones H. 2004. Hydrellia pakistanae and H. balciunasi, insect biological control agents of hydrilla: boon or bust? In Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (editors). Proceedings of the XI International Symposium on Biological Control of Weeds, 27 April - 2 May 2003. CSIRO Entomology, Canberra, Australia.
- Grodowitz MJ, Center TD, Cofrancesco AF, Freedman JE. 1997. Release and establishment of Hydrellia balciunasi (Diptera: Ephydridae) for the biological control of the submersed aquatic plant Hydrilla verticillata (Hydrocharitaceae) in the United States. Biological Control 9: 15-23.
- Holm L, Doll H, Holm E, Pancho J, Herberger J. 1997. World Weeds: Natural Histories and Distribution. John Wiley, New York, NJ.
- Johnson NF. 2014. Hymenoptera Online (HOL). Available online: http://hol.osu.edu/index.html?id=16972 (Accessed 23 June 2020)
- Langeland KA. 1996. Hydrilla verticillata (L.f.) Royle (Hydrocharitaceae), the perfect weed. Castanea 61: 293-305.
- Lawton JH. 1985. Ecological theory and choice of biological control agents. In Delfosse ES (editor). Proceedings of the VI International Symposium on Biological Control of Weeds, 19-25 August 1984. Agriculture Canada, Ottawa, Vancouver, Canada.
- Wheeler GS, Center TD. 2001. Impact of the biological control agent Hydrellia pakistanae (Diptera: Ephydridae) on the submersed aquatic weed Hydrilla verticillata (Hydrocharitaceae). Biological Control 21: 168-181.